Electrolysis is the act of driving a chemical reaction with DC current. Inside a simple electrolytic cell, current usually passes through a conductive liquid to cause a chemical reaction to take place. Electrolysis of fresh water is used to produce the pure gasses hydrogen and oxygen. The important ‘chlor-alkali process‘ or electrolysis of sea water might be preformed to produce chlorine and sodium hydroxide or even sodium hypochlorite or sodium chlorate. If a solution contains metals then electrolysis can be used to isolate and to purify those metals or to coat an object with a metal. This process is called electroplating or electrophoretic deposition. A chrome automobile bumper and a galvanized item that was neither “hot dipped” nor “peen plated” are classic examples of commercial electroplating. In both cases the coating metals (chrome or zinc) act to protect the underlying ferrous metal from the results of nature’s own electrochemical reaction, rust. Anodizing as well is a process that uses electrolysis to increase corrosion resistance. The body of an anodized aluminum flashlight for example, was dipped in an electrolytic cell in which the polarity is reversed from normal electroplating. While normal electrolysis is often used to remove rust from antique artifacts, rust patinas can actually be deposited upon items like roof sheathing or automotive parts using the reverse polarity – anodizing process. Galvanization, electroplating and anodizing are preformed daily by industry but can also be accomplished at home on a smaller scale.
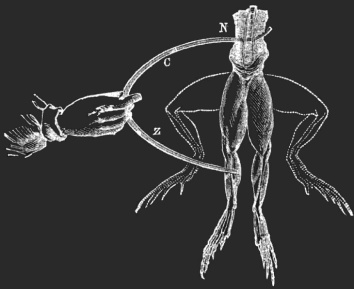
Shortly before the American and French revolutions two contemporary Italian professors were having a disagreement about what electricity was and where it came from. In about 1771 Luigi Galvani was dissecting a frog in search for its testicles. Luigi picked up a scalpel and probed about, only to be startled when the dead frog’s leg twitched or convulsed. He was to later attribute this reaction to some kind of “animal electrical fluid “. Alessandro Volta later argued that this physical phenomenon was caused by an electrochemical reaction from dissimilar metals. Volta actually invented the first battery – the “voltaic pile” to demonstrate his idea and to disprove Galvani’s theory. Volta later and perhaps sarcastically coined the term “galvanism” for direct current produced by chemical reaction.
The symbol for a battery is a stylized representation of a voltaic pile. Volta’s battery was a stack of alternating copper and zinc disk separated by paper soaked in saltwater. One of the most popular introductory scientific experiments for school children is to construct a voltaic pile using a stack of copper coins and aluminum foil – separated by paper and with an electrolyte of lemon juice or brine solution.
* An electrolyte is any substance that contains free ions – which makes the substance electrically conductive. Normally electrolytes are liquid but occasionally can be either gaseous or solid too.
* Another contemporary of Galvani and Volta was the well traveled Benjamin Franklin who spent several years in Europe as an American diplomat. Franklin coined the term “battery” in 1748, before such a working device had been invented. Franklin somehow associated a line of connected Leyden jars crated in a tray – with a line or battery of cannon protruding from the side of a warship. In his famous kite and lightning experiment Franklin actually used ‘Leyden jars’ which were essentially capacitors. Leyden jars had only been invented, independently, a couple of years beforehand (1745-1746) by a German and a Dutchman. Leiden is a city in the Dutch province of South Holland. The term “battery” properly applies not to one, but to a group of connected galvanic cells.
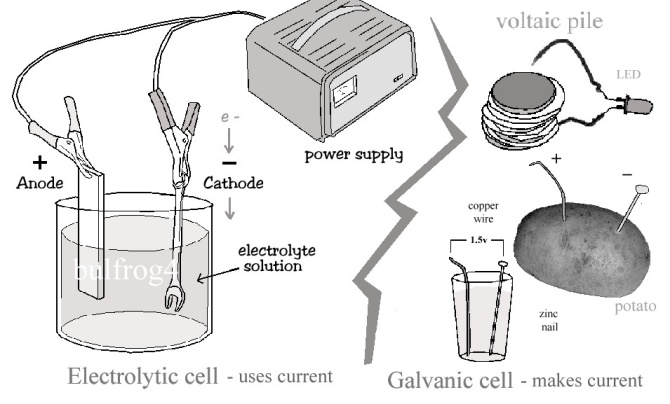
* In a simple galvanic cell chemical energy from the ionization of different metals is converted to electrical energy. Conversely, in an electrolytic cell, voltage is applied to the electrodes and electrical energy is converted to chemical energy. In a single cell of a rechargeable lead-acid (automotive) battery or a rechargeable NiMH / NiCD flashlight battery, the cell acts as a galvanic cell when discharging but as an electrolytic cell when being charged. The difference between a “primary” battery and a “rechargeable” battery is that the chemical reaction is easily reversible in the latter.
* Confusion exists between the terms anode and cathode. In an electrolytic cell the anode is positive (+) and the cathode is negative (-). Negative ions (anions) are attracted to anode; positive ions (cations) are attracted to the cathode. In a voltaic cell however the polarity of cathode and anode are reversed. This discrepancy is attributable to the theory that an anode should release electrons and undergo oxidization, whereas the cathode should undergo reduction.
* Oxidation and reduction are opposite reactions that occur naturally in the atmosphere or at the anode and cathode during electrolysis. Rust is caused by iron giving and oxygen taking electrons – the iron oxidizes or looses some of its surface electrons to atmospheric or surrounding oxygen which is very reactive and ever hungry for more electrons. Reduction is the gain of electrons or a decrease in the oxidation state of an atom.
In the rust removing electrolysis process water is oxidized at the anode, oxygen is produced as the anode corrodes and ions or salts of metal slowly enter suspension within the electrolyte. At the negative terminal – the DC power source supplies electrons to the cathode where reduction occurs (water and rusty item accept electrons or ions) and lots of little hydrogen bubbles are produced.
Acids like vinegar (acetic acid), hydrochloric or phosphoric acid can be used to remove iron oxide from rusty items. Acids in this application however can be destructive to rusted antiques and artifacts and this is where simple electrolysis might be preferable. Many carbonated soft drinks will remove rust to a degree also. Some of the CO2 in solution turns to H2CO3 (carbonic acid) which reverses oxidation (reduction). Some Cola (s) also contain phosphoric acid (H3PO4) which is an active ingredient used in the steel industry to ‘pickle steel’ or in the rust removing gel called ‘naval jelly’.
There are in essence, two kinds of rust. Red rust exfoliates or expands. Red rust (ferric oxide/ Fe2O3) is lost or unsalvageable but at least it can be loosened and freed up by the action of hydrogen bubbles in the electrolytic process. Underneath the red rust is “black rust” (or magnetite / Fe3O4) which is still strongly bonded to the underlying metal. Black rust can be reclaimed and reduced back to metallic iron. Any deep pits beneath a badly rusted surface will not be filled up, but the surface magnetite will be stabilized – its molecules invigorated with new electrons to reform iron. After drying off, rusty objects cleaned this way will quickly regain rust so therefore should immediately be protected with oil, paint, varnish or something similar.
* Using a ferrous metal sacrificial anode which contains stainless steel, copper, zinc or nickel might cause some plating of those metals onto the cathode. Stainless steel contains chromium and should be avoided for use at the anode because poisonous gasses and dangerous chromates could be produced in the electrolyte. This electrolysis process also produces small amounts of pure oxygen and hydrogen so the project should be preformed in a well ventilated area and any sparks or flame should be avoided.
Since fresh water is such a poor conductor of electricity it needs to be amended with other substances before it will conduct, and therefore participate in electrolytic ion exchange. Acid solutions or salt water would work, but these are corrosive and less desirable than using alkaline solutions for a rust removing electrolyte. Any base like sodium bicarbonate (baking soda), sodium carbonate (soda ash), potassium carbonate (potash), sodium hypochlorite (laundry bleach), sodium hydroxide (lye or caustic soda) or perhaps even ammonia can be added to water to make a good electrolyte. The concentration of the base in the liquid is apparently not critical, anywhere between 2% and 10% should do.
The DC power can come from almost any source depending upon the size of the project. Flashlight batteries, car batteries, power supplies salvaged from old PCs, automotive battery chargers and even DC arc welders have been used. Where delicacy is called for when restoring a historical artifact about the size of a horseshoe, a 12v or 6v battery charger even on its lowest setting might produce too much amperage. To place denser, less porous deposits on small cathodes (items) a slower working current of perhaps 200mA @ 12 V is more appropriate. For larger items more amperage can be applied; more amperage means faster electrolysis. DC arc welders work at about 40 volts and put out somewhere between 75 and 185 amps to make an 1/8th inch rod melt. Although they produce high amperage arc welders don’t put out enough voltage to pose a life threatening risk from electrical shock.
There is no point to placing a clean unblemished piece of iron or steel into such an electrolytic cell because nothing will happen when using this simplistic type of electrolyte. Unlike electroplating gold onto a coin or jewelry base metal, this example of electrolysis using a mild alkaline electrolyte will not properly deposit new iron onto the cathode.
* As a footnote, electrolysis is important in industry because valuable chemicals and metals can be produced, isolated or concentrated including: aluminum, copper, lithium, sodium, potassium, magnesium, chlorine, sodium & potassium chlorate and sodium hydroxide. Fuel cell cars of the future would depend upon hydrogen, largely from production by electrolysis. Modern nuclear submarines are capable of remaining submerged for extended periods because electrolysis can be used on water – to extract pure oxygen, which is then mixed with “scrubbed” and recycled air.
* There is another definition of “electrolysis” which is concerned with hair removal. This association might have been made because doctors, beauticians and cosmetologist originally tried to kill hair follicles with electrified tweezers. Today it is more common to see tiny laser beams being used to permanently remove hair.
Reverse electrolysis can be used to put rust on ferrous metal. As with anodizing the very same electrolytic principle is at work but the polarity of contacts is reversed. Rusty patinas can be deposited upon shiny new automotive or mechanical parts to make them match the look of old engines or original machinery. Rusty patinas can be put on new corrugated galvanized roofing metal to make the roof of a house or barn look more ascetic. Anodizing as well employs reverse electrolysis and gets its name because the object to be modified works as the anode in the electro-chemical reaction. Aluminum especially but titanium, zinc, magnesium, niobium and tantalum are examples of metals that can be anodized and benefit from the oxidization process. Aluminum normally “self passivates” or builds up its own microscopic oxide surface layer very quickly, which protects it from further corrosion. Chrome, magnesium, titanium and zinc do the same thing. In the act of oxidizing these metals rather than trying to plate them, a corrosion resistant protective layer is created on the surface that prevents further oxidization. The anodizing process actually makes the surface tougher, less conductive, more porous than normal and therefore capable of absorbing dyes or holding paints and lubricants longer.
Magnesium is often anodized in dichromate solutions. Titanium can be anodized in phosphoric acid (remember cola soft drinks) or an alkali solution like trisodium phosphate (TSP / dishwasher detergent). Zinc is rarely anodized but here again a solution of phosphoric acid might produce good results. Aluminum which is frequently anodized is perhaps best preformed upon using a mild sulfuric acid electrolyte. Colored dyes like those from the ink in a permanent marking pen can be used to tint a freshly anodized object. A protective coating of lacquer or plastic polymer will further protect the dye.. <Long winded but thorough video on simple anodizing.>
Galvanization is the process of putting a protective coat of zinc on steel (normally). When steel is galvanized, oxygen reacts with the zinc to form zinc oxide and then that reacts with carbon dioxide to form a very corrosion protective zinc carbonate surface. There are three common ways to apply a galvanized coating: electroplating, hot dip and mechanical plating. A piece of steel that has been electroplated sometimes shines like stainless steel, but is less magnetic. A piece of steel that has been hot dipped usually has a dull mat-grey surface, exhibiting a crystalline spangle. “Galvanization” should refer to an electrochemical process (considering Luigi Galvani’s name) but “hot-dip galvanization” is not an example of electrodeposition. Instead, steel is dipped into a molten bath of zinc (@ 860 ºF apx.). This creates a metallurgical bond with the underlying metal and microscopic layers where the zinc and iron are alloyed. A third type of galvanization first appeared in the 1950’s. Mechanical plating (or peen plating, mechanical deposition, or impact plating) essentially puts small items into a large tumbler and beats them around in the presence of glass or ceramic beads, water and a zinc powder. Many nuts, bolts, washers, springs, clips, nails and screws are treated in this fashion. Each of the galvanization process has advantages and disadvantages. Electro-galvanization might be applied to automobile bodies and pneumatic nails and brads but the coating is not as robust nor as protective as a hot dip treatment. The hot dip process might weaken the strength of the base metal or fill up the screw threads of a nut and bolt. Impact plating only works well for smallish, non flat items.

Zinc.photo © by Heinrich Pniok (www.pse-mendelejew.de) license: http://artlibre.org/licence/lal/de
Small scale electro-galvanization is easily achievable at home. Parts to be plated should be washed with soap and water and then perhaps etched in a dilute muriatic (hydrochloric / swimming pool) acid bath. A useful zinc electroplating solution can be made from the zinc oxide powder 2 parts and lye (sodium hydroxide) 10 parts for every 128 parts of water. A splash of glycol (as in automotive antifreeze) supposedly makes a brighter finish. Another zinc electroplating solution consist of about 3 parts zinc chloride and 12 parts ammonium chloride with 100 parts water (by weight). Yet another zinc plating electrolyte would be white vitriol (the historic name for zinc sulfate heptahydrate). Zinc sulfate can be purchased or made by subjecting zinc metals, minerals or oxides to sulfuric acid. This video named “Turn Pennies Silver & Gold” demonstrates electro-galvanization using zinc sulfate.
A pure zinc metal anode might be a little hard to come by. Usually alloyed with other metals, zinc is not rare but neither is it used often in a pure state. Scraps of hot plated galvanized metal could serve temporarily as anodes. Again, when electroplating the positive (+) wire goes to the zinc anode and the negative (-) wire to the item to be zinc plated.
* Since 1982 American pennies contain 97.5% zinc but the slugs are electroplated with copper (2.5%) before they are stamped. Incidentally, by virtue of its intrinsic metal the most valuable U.S. coin in common circulation – is the 5 cent nickel.
Plating applies a deeper more durable layer than simple deposition does. Electroplating a thick layer of a particular metal onto the cathode generally requires a sacrificial anode of the desired metal and an optimized electrolyte containing salts of that metal suspended in solution. Increasing the temperature of the electrolytic bath will improve plating efficiency. Oftentimes these optimized electrolytes are very caustic or poisonous. Quality, industrial grade electroplating is outside the realm of home experimentation not only because of the cost but because of the toxicity of the chemicals involved. There are small commercially purchasable “kits” available for electroplating copper, gold, silver, nickel, brass, chrome, black chrome and zinc-cadmium. It must be presumed however that these consumer grade kits are less effective for electroplating than are those cyanide processes that industries are regulated by license to use.
Copper plating can be achieved in an acid sulfate, alkaline or cyanide bath. Cupric sulfate (copper(II) sulfate or CuSO4) is easily made or is purchasable from an Arts & Crafts store or in the form of a solution poured into septic lines to kill tree roots. Using cupric sulfate as a copper plating electrolyte works to a degree but the end result will be a thin, duller and less bright finish than when using an industrial cyanide solution. Adding soap or glycol to the safer cupric sulfide electrolyte solution might help improve the luster of the plated object. A simple bath in copper sulphate (no current) of somewhat reactive metals like aluminum and zinc will cause deposition of some copper upon those metals.
* Cupric sulfate is also used to kill and control algae in swimming pools or aquariums, to dye organic fibers and to etch zinc plates in one form of printmaking. Relatively benign but somewhat toxic it can irritate the eyes and skin and should not be swallowed. Copper(II) sulfate can be made industrially by soaking copper in hot concentrated sulfuric acid or as the image below shows; by a hobbyist using a battery charger, two electrodes of copper and a sulfuric acid electrolyte.
* Nobel metals are less reactive than most other elements and are resistant to corrosion and oxidation. Nobel metals include: gold, iridium, osmium, palladium, platinum, ruthenium, rhodium, and silver (by some accounts mercury, rhenium or copper might be included within this list). The main eight noble metals are also “precious” but precious metals by definition are rare, valuable and occur naturally.
Cyanide is compound built upon a carbon and a nitrogen atom where the two have been triple-bonded to each other. Extremely important to industry on several levels, not all cyanides are toxic. Algae, fungus and bacteria produce some types of cyanide but it is the cyanide compounds that release negative cyanide ions (CN-) that are dangerous. Both sodium and potassium cyanide act like solvent upon the noble metals and they have been used historically to dissolve, leach and extract gold, silver and copper from ore. It is their high affinity for metal that makes sodium and potassium cyanides so valuable to the mining process or for electrolyte solutions in the electroplating process. Both are created by treating their hydroxides with hydrogen cyanide (ex: hydrogen cyanide + lye (sodium hydroxide) = sodium cyanide …. hydrogen cyanide + caustic potash = potassium cyanide).
Over 2.02 billion pounds of hydrogen cyanide was produced and used in the U.S alone in 2003. The chemical is important for pharmaceuticals, plastics, acrylics, leather tanning and other things. Hydrogen cyanide (HCN or prussic acid) is primarily produced by the Andrussow Process where methane and ammonia are reacted in air over a platinum catalyst at high (1100° C) heat, but there are several other ways to produce it. Potentially lethal, HCN and some other cyanides are some of the fastest acting poisons known to man and can kill by blocking electron transport, therefore stopping respiration. HCN was the most lethal chemical gas used on the battlefields of WWI and has been used to execute both rodents and people for more than a couple of centuries now. Although very much ‘on the radar’ of organizations like the EPA, cyanides used in mining operations do not have a high persistence and those exposed to sunlight degrade fairly rapidly.
Chromium is a metallic element (Cr, atomic # 24) that is very hard and corrosion resistant. Chromium has a very high (3465 °F / 1907 °C) melting point that is higher that that of both kaolin and platinum. Stainless steel contains some chromium. Although not officially identified until 1798 it appears that about 2,000 years ago Chinese metallurgist were dipping sword points and arrowheads into chromium. Prior to the 1920’s chromium was pretty much only used for paint pigments or for leather tanning solutions. Clock parts and shiny car parts were primarily electroplated with nickel before chromed parts arrived on Ford and Chevrolet automobiles around 1926.
Aside from regular steel, chrome plating can be applied to stainless steel, brass, copper, aluminum and even conductive plastic. Scratch removal and thorough cleaning are critical in chrome plating preparation. For steel, usually a layer of copper and then nickel are first deposited before the chrome layer even goes on. The quality of these two layers is important because chrome is brittle and its layer is usually quite thin. The most lustrous, most reflective and attractive chrome plating also involves using the most toxic process – called hexavalent chromium plating. In a hexavalent chromium bath carcinogenic sodium chromate (Na2CrO4) or dichromate (NaCr2O7) are suspended in hot sulfuric acid to make the dangerous electrolyte called chromium trioxide (or chromic anhydride). Listed as a “priority pollutant” by 1977 in the U.S., every drop of this chromic acid is accounted for and tracked. Automobile manufacturers in the EU stopped using “hex-chrome” in 2006.
The name “hexavalent” refers to the chromium molecule being in its +6 oxidization state. Likewise the name trivalent reflects the valence of chromium. “Tri-chrome” or trivalent chromium plating is a newer, alternative chromium plating technique. Tri-chrome uses a far less toxic electrolytic bath and sophisticated anodes but the process is even more complicated and the finish sometimes inferior to Hex-chrome.
Although used as a precursor to chrome plating, nickel plating may not be as widespread in industry as it once was. Simple nickel plating can be accomplished by an amateur using a salt, acid or alkaline electrolyte bath. Of the various combinations published for nickel electrolytes, the nickel sulfate, nickel chloride and boric acid mixtures (together in unison) seem to be the most mentioned. <Video of simple nickel plating.>
Electrolysis, galvanization, electroplating and anodizing all fall within the realm of arts and crafts or home fabrication. The quality of the resulting work will sometimes be determined however by the cost and dangerousness of the materials applied. Copper, gold, platinum and silver plating for example might require costly ingredients and potentially hazardous hydrogen cyanide derived electrolytes. The complications of quality chrome plating remove this process from the general capability of the private individual.
———–
Added: Oct/12/2013
Industry standard colors for wires coming out of the back of an ATX type PSU (Power Supply Unit) like this one are: red > for + 5v DC, yellow > for + 12 v DC and black > for ground. A few other colored wires (orange, blue, grey or green) were unimportant and therefore isolated and tucked away, back inside the box. Computer PSUs like this are “switchmode” (or switching mode) power supplies which means they might need a “dummy load” to work correctly. Rather than use a 10 ohlm/10watt wire wound load resistor that some sources suggest, this jury rigged example uses a little automotive light bulb. Housed in a red, trailer running type light fixture, the bulb provides both a power on indication and a dummy load for the switching mode power supply. One red (+5v) and one black (GND) wire are connected to the bulb. The black circular receptacles were originally speaker wire terminals scavenged from the back of some old speakers. On the left, some extra yellow and black wires await attachment to an automotive type cigarette lighter receptacle. Any accessory that normally plugs into a car’s cigarette lighter could easily be powered by 12 volts from this PSU.
In this picture a jar of blue, recently homemade copper sulfate sits in the background. Two copper electrodes dipped in a solution of about 15% sulfuric acid / 85% water turned a clear solution to that blue color in a couple of hours using 5v DC. The solution which contains copper ions will be used to test some simple copper electrophoretic deposition on different metals.
Currently working in the coffee cup is a steel nail and a rusty razor blade that has been temporarily pulled up out of the solution. The electrolyte in the cup consists of ½ cup water and 1 tsp laundry bleach. White bubbles from the razor cathode and brown rusty flotsam from the sacrificial nail anode obscured the originally clear electrolyte rather quickly. Within the same cup but clean solution, one or several keys could be accommodated and anodized instead. By reversing the polarity a rough patina could quickly be etched upon keys containing zinc, which in turn would allow better adherence of indelible inks (as from multicolored permanent marking pens) for easy identification.






I really got into this article. I found it to be interesting and loaded with unique points of interest. I like to read material that makes me think. Thank you for writing this great content.
so much great information on here, : D.
Just wanna input that you have a very nice web site, I enjoy the design it really stands out.
I like your website, it has awesome content.
For most excellent contents, go to see this
web page daily because it offers quality.
Keep working ,impressive job!
I’m typically to running a blog and i actually respect your content. The article has really peaks my interest. I’m going to bookmark your website and preserve checking for new information.
There is only one “guy” responsible for this blog (me); and he has not been paying much attention to it lately.
Does your site have a contqct page? I’m having a tough time locating it but, I’d like
to send you an e-mail. I’ve got some creative ideas
for your blog you might be interested in hearing. Either
way, great website and I look forward to seeing it grow
over time.
This comment looks like simple spam of the type I have deleted on many occasions. Nonetheless I could amend the blog introduction somehow by adding at least an e-mail address. I can be contacted by writing to cactusbush@gmail.com . Something like the keyword “blog” added in the subject line would be useful to get my attention.
Hi . I just want to know what will be the galvanic effect between anodised aluminium and Low Carbon Steel & Effect between Stainless Steel and Anodised Aluminium. Please support. I’m asking this as your blog is so impressive
This is a excellent blog, would you be involved in doing an interview about just how you designed it? If so e-mail me!
Great site. Thank you for posting
I was just looking for this info for a while. After 6 hours of continuous Googleing, at last I got it in your site. I wonder what is the lack of Google strategy that don’t rank this kind of informative websites in top of the list. Generally the top websites are full of garbage.
Hello,
I am looking to remove rust from an etching plate. There was a picture on Google of a pheasant etching being cleaned. I was not able to find it on your blog. Any chance you could help me out with tips or your original posting? Thanks.
You’ve given no information about the metal involved. Mild acids like phosphoric, oxalic, citric or acetic – might prove useful.
i love your content information seems overwhelming following this cool website i love your content
Pingback: URL
This is very good.
Thank you
Colin
How to galvanize large things like a bunch of pipe for a fence? WHat kind of current do I need, chemicals etc for something 6 feet long. Its much cheaper to buy non galvanized pipe and wire.
Thanks
You could electro-deposit zinc onto steel pipe but the final result would be thin and eventually ineffective for a fence post. For something that is meant to stick into the ground, this method would be ill advised. ‘Hot dipped’ galvanization deposits a much thicker layer of rust proofing. If you were determined to electroplate a large piece of steel with zinc you would simply need a large water proofed tub or trough, a mild water/ bleach electrolyte, an adequate source of zinc for the anode and a large DC current like that provided by a DC arc welder. This link from the article (http://antique-engines.com/trailer-electrolysis.htm) describes removing rust from a large trailer frame. The same process can be turned around for electro-galvanization.
A useful zinc electroplating solution can be made from the zinc oxide powder 2 parts and lye (sodium hydroxide) 10 parts for every 128 parts of water. Another zinc electroplating solution consist of about 3 parts zinc chloride and 12 parts ammonium chloride with 100 parts water (by weight). On Amazon.com – zinc oxide powder cost about $6 a pound, ammonium chloride cost about $7 per pound and zinc chloride the most expensive at perhaps $40 / lb.
Pingback: Purification by fire | the tao of jaklumen
Awesome things here. I am very satisfied to peer your post.
Thank you so much and I’m having a look ahead to contact you.
Will you please drop me a e-mail?
One that provides the reader with a general understanding of what your blog post will be about. If so you might want to consider joining some blog hops or “memes” as they are sometimes referred to.
As a corporate leader, it is easy to get carried away with giving information and advice to others through a blog. However if you have multiple authors or a corporate blog, the secondary software may become necessary.
text excellent – very good 2
como reconquistar http://www.reconquistar2.com/
Pingback: funktion one speakers for sale
Pingback: Fat loss factor doesn't work that well
Pingback: ab exercises video
Pingback: Wedding Photography Adelaide
Pingback: website info
Pingback: contesting my wifes will
Pingback: click the loft site
Pingback: Yeast infection home remedy
congratulations all for the excellent text – very good
Pingback: Homepage
Pingback: Hot Stuff 2 | cactusbush
Pingback: Hot Stuff – metal, ceramics & glass | cactusbush
If you could e mail me with a few hints on just how you made your blog look this excellent, I would be thankful.
clash of clans cheats http://www.supercheats.com/iphoneipad/clashofclans.htm
Genius Who Will Be Scared Of men.
???
Your blog are impressive to each other.I read your blog its very good and friendly, Help ful for all.
Zinc Nickel Plating Process.